Piping materials to optimise PEM electrolysers
Only the best electrolyser OEMs, using the best components, are able to out-perform their rivals on multiple performance attributes. Component-level choices matter. And on top of that, there must be a cohesive concept which integrates the holistic design, from feed water entering the system to pure, pressurised hydrogen leaving it.
Building an excellent electrolyser is like cooking a great meal: choose only the absolute best ingredients and cook them to perfection. The components that are incorporated into the stack, and balance of plant are the key ingredients. Good system design and high-quality manufacturing must then follow.
Given the number of components in an electrolyser system, and the multiple options for each element, there are thousands of degrees of freedom that an electrolyser OEM can leverage to optimise their product for cost, efficiency, dynamic response, or durability.
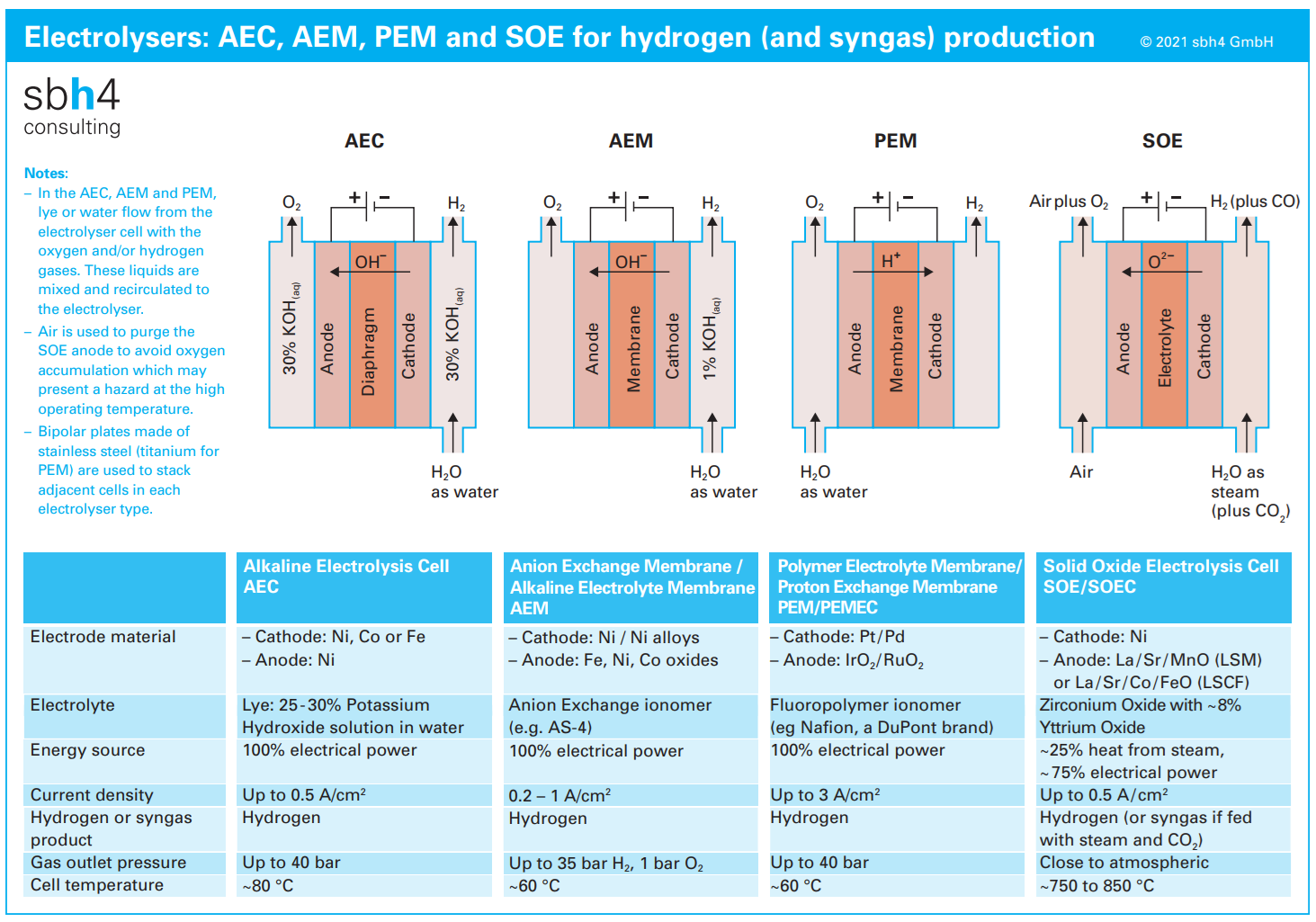
Electrolyser technology categories
There are four broad categories of electrolyser technology for hydrogen generation.
Alkaline water electrolysis (AWE) operates using an electrolyte which contains about 30% by mass of potassium hydroxide (KOH). This is referred to as lye and is an extremely alkaline liquid.
Anion exchange membrane (AEM) electrolysers also use an alkaline electrolyte. In comparison, to the AWE technology, the AEM electrolyte will either have a lower concentration of KOH or use a benign bicarbonate solution.
Proton exchange membrane (PEM) electrolysers use pure water at the electrolyte. During electrolysis, an acidic environment is created. This is a key differentiator between PEM electrolysers and the AWE and AEM classifications.
Solid oxide electrolyser cells (SOECs) use a solid electrolyte. Their design and operation is vastly different to AWE, AEM and PEM systems, which rely on liquid electrolytes. Furthermore, SOECs generally operate in the temperature range 700 to 900 °C – significantly higher than AWE, AEM and PEM technologies which operate in the range of 60 to 120 °C.
Acidity is unavoidable in PEM systems
Materials selection for AWE and AEM electrolysers operating in an alkaline liquid is different to the case for PEM. Additionally, the AWE and AEM stacks are more robust than PEM stacks meaning they are more tolerant of some impurities in the electrolyte.
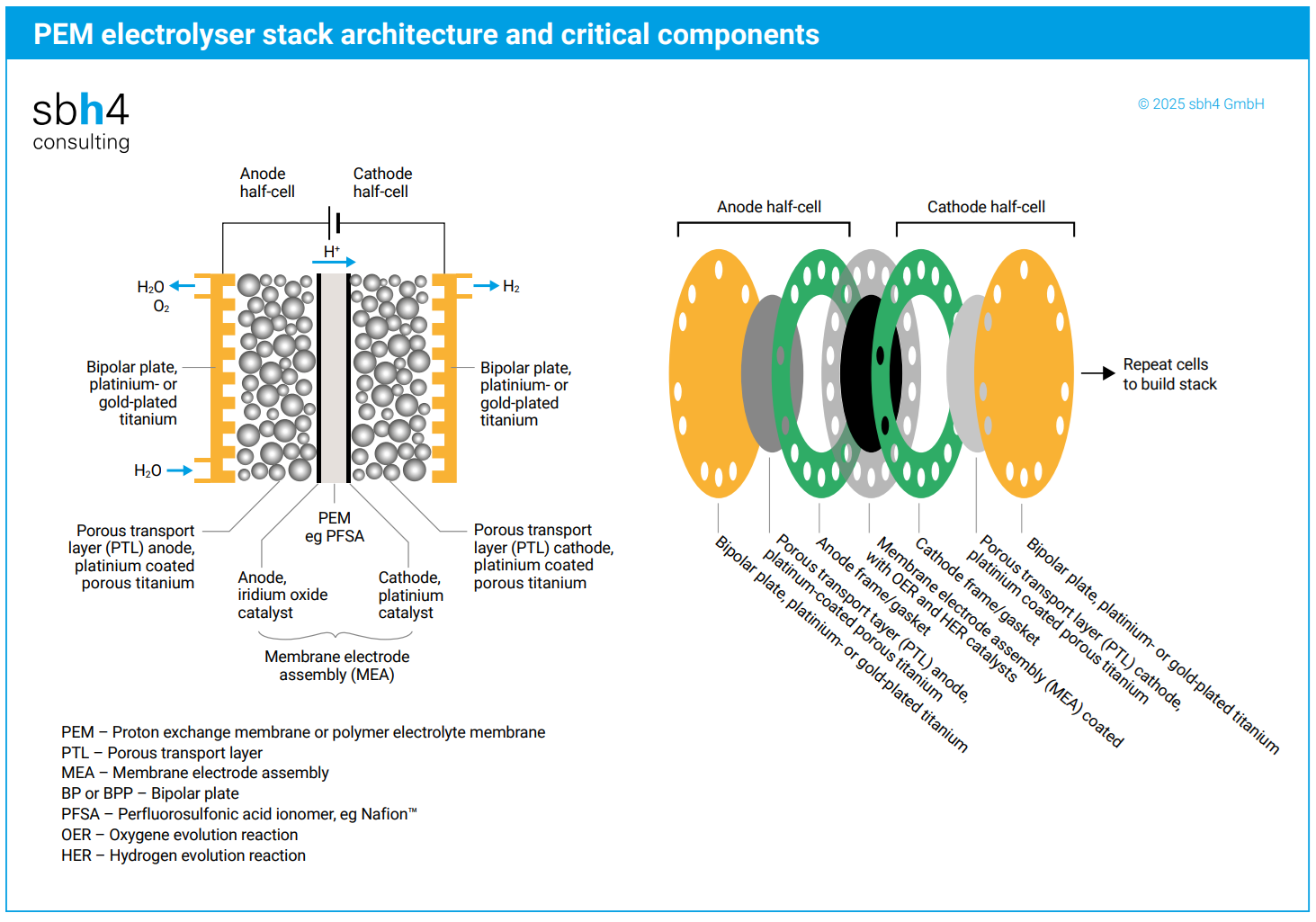
The PEM membrane must be manufactured from an extremely resilient polymer to avoid it being damaged by the acidity surrounding it. A fluorinated, sulphonated polymer called perfluorosulfonic acid (PFSA) is the default choice to achieve this goal.
Using a fluorinated polymer is a double-edged sword. On the one side, the fluorine makes the polymer robust. On the other side, the acidity in the PEM stack is so extreme that some fluorine is released from the PFSA membrane as hydrofluoric acid (HF). HF is one of the most aggressive acids known to chemistry. Over time, HF attacks the highly sensitive PEM electrolyser’s proton exchange membrane, which is intentionally very thin to reduce ohmic losses.
Durable PEM membranes
A PEM stack with a PFSA membrane is inherently subject to self-degradation over time. The challenge is to balance the design to achieve several years of stable operation at high efficiency before the stack deteriorates to such an extent that the electricity input required to generate hydrogen rises to such an amount that re-investment in new stacks is cost-effective.
Avoidance of HF would be possible if an alternative membrane were to be used. Selection of a membrane that avoids fluoropolymers would also reduce concerns related to ‘PFAS’: per- and polyfluoroalkyl substances which are known as ‘forever-chemicals’ which are harmful to the environment and extremely difficult to break down in nature.
Toray, and several other companies are working to develop fluorine-free, PFAS-free PEM membranes. However, at present, the state of the art relies on PFSA, due to its resilience.
Feed water purification
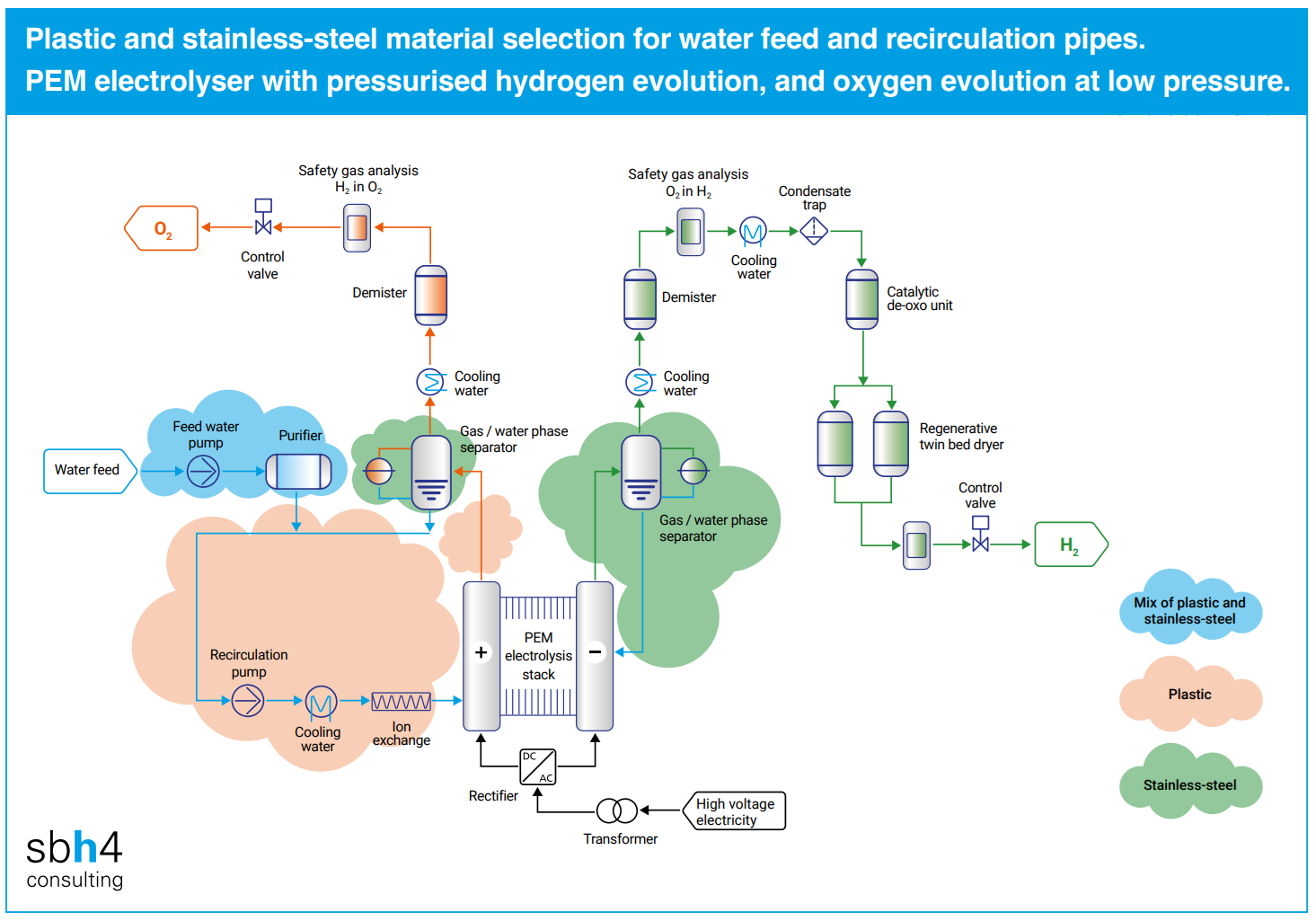
The feed water to the electrolyser must be purified to an extremely low level of ionic conductivity. The purification equipment for this can include ultrafiltration, reverse osmosis, and ion exchange resin beds.
However, even the most robust purifiers allow a few parts per billion of contaminant ions such as calcium, magnesium and iron being introduced to the electrolyser. The presence of iron oxide particles in the size range of 0.1-5 µm in the electrolyser recirculation water can be observed through ‘rouging’. Rouging is caused when pure water at around 60 °C comes in contact with stainless steel.
Iron oxide and other metallic ion impurities are very damaging to the membrane. Big cations such as calcium and magnesium are membrane killers because they block the proton transfer ‘pores’ in the membrane.
Blockages in the proton exchange membrane lead to a progressive increase in ohmic resistance. In turn, that means a reduction in the stack efficiency and an increase in electricity consumption per kg of hydrogen produced. This is a core reason for PEM stack degradation.
Limitations of stainless steel
Selection of stainless steel for water purification system and PEM electrolyser pipework is common because it is strong and releases fewer ions into the water than carbon steel. However, even the highest grades of stainless steel can release metallic ions into the feed water and circulating water.
Specification of drawn stainless steel tubing with internally polished surfaces is essential if this material is used in PEM electrolysers and ultra-pure water treatment systems. This is potentially a limitation to the scale up of PEM electrolyser stacks because this type of pipework is only available at smaller diameters.
The use of large-diameter stainless steel pipe, fabricated with a welded seam would be fatal for a PEM electrolyser. At a microscopic level, the weld is rough and porous and may be a catastrophic source of ionic contamination. Similarly, when joining stainless steel piping sections, welds must be avoided.
The case for plastic pipework
An alternative approach to minimise the potential for damage to the membrane is to minimise the use of metallic pipework in contact with the feed water and ultra-pure water in the electrolyser recirculation circuit. With fewer impurities reaching the membrane and catalysts, the stack degradation rate is reduced and its usable lifetime is extended.
Similar to the many grades of steel and stainless steel that might be selected, there are many types of plastic that are used to construct pipes. Not all of these are suitable for use in PEM electrolysers. Only appropriate, sophisticated engineered plastics should be used.
Engineered plastics
There are many different plastics that we encounter in our daily lives. Polyethylene (PE) is commonly used to make bags and food packaging. Polypropylene (PP) is denser and is preferred for car parts and sturdy children’s toys such as a garden slide. Applications of Polyvinylchloride (PVC) range from construction materials for window frames to medical devices.
Engineered plastics differ from household plastics due to their enhanced properties of temperature, pressure or chemical resistance. When designing a PEM electrolyser, superior performance in all three of these attributes is essential.
The most suitable grades of PP and PE for use in ambient temperature water purification systems in electrolysers are PP-H and PE100. These are more suitable for high purity applications than regular PP and PE piping, because the manufacturing process ensures very smooth internal wall surfaces and virtually no extractable ionic or particulate contamination. For the electrolyser water recirculation PP-H is preferred because it is more stable at the elevated temperature of around 60 °C.
Pressure limitations
Many PEM electrolysers produce hydrogen at an elevated pressure between 20 and 40 bar. A notable exception to this is Siemens Energy’s Elyzer-P which operates close to atmospheric pressure. The oxygen side of PEM systems can either be at a low pressure in the order of 3 bar, or at an elevated pressure similar to the hydrogen side of the system.
There is a trade-off between temperature and pressure when using thermoplastic plastic components: at higher temperatures, the maximum safe working pressure is reduced. PEM electrolysers generally operate at around 60 °C and the maximum pressure rating of PP is reduced by half at this temperature compared to the allowable pressure for operation at 20 °C.
Highly stable fluoropolymer piping
Moving beyond the water treatment system, PVDF and ECTFE are highly stable fluoropolymer materials. ECTFE has some advantages over PVDF, namely it is less susceptible to chemical stress and can be used at higher temperatures. These characteristics make it ideal for electrolyser piping circuits in PEM electrolyser systems.
PVDF and ECTFE may be suitable for use in the condensate water return line from the oxygen phase separator at pressures of up to around 10 bar. They are both non-corrosive and minimise iron ion release. This avoids rouging of the recirculation water and damage to the stack.
***
Author credit – Stephen B. Harrison, sbh4 consulting.
sbh4 is an independent advisory firm focused on decarbonisation and defossilisation through e-fuels, e-fertilizers, biofuels, SAF, CCTUS, GHG emissions reduction, and the emerging hydrogen economy. For more information, visit www.sbh4.de.