Q&A with HYDErogen part 3
Under the spotlight: Hydrogen electrolyser technology part 3
Continuing our interview with guest contributor Dr Kris Hyde, we spoke to him about the basics of hydrogen electrolyser technology and the challenges faced from the various options available. This time, the focus is on operating efficiencies.
Dr Hyde, a specialist in electrolysis technology, is director of HYDErogen, a consultancy firm offering technical and commercial advice on hydrogen-related technology and business.
Why does electrolyser efficiency vary with percent output?
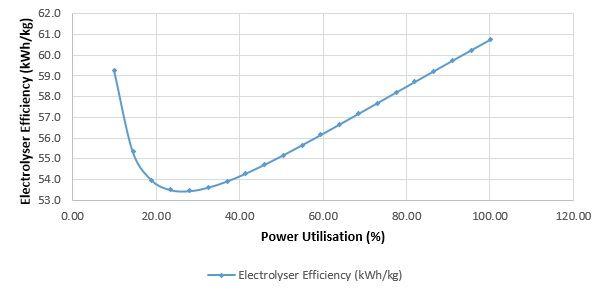
Electrolyser manufacturers often quote two key numbers: The peak flow and the efficiency - this is usually the peak efficiency, but it's not possible to have the peak flow at the peak efficiency.
The graph belows shows hypothetical data for the kWh/kg (electricity consumed per kg of H2 output) for a typical PEM electroylser. Note this is not an efficiency curve, it is an 'inefficiency curve', so lower kWh/kg is higher efficiency.
Image courtesy of HYDErogen
Points to note:
The highest flow rate is at the most inefficient point of the curve. If you run your electrolyser hard to 'sweat your asset', you're running it inefficiently
The most efficient point is at ~30% (this varies between electrolyser models, so don't take this value as gospel – it’s the general shape of the curve here that is important). Here, it is 10-15% more efficient than peak output. This is due to the electrochemical nature of electroyser cells - the higher the current you push through them, the higher the voltage and the lower the efficiency.
The inefficiency rapidly rises below this point. This is due to the fixed power consumers on the plant, (pumps; though usually on an inverter, control systems, heaters, fans, chillers, valves, sensors, de-oxo units, etc). At lower outputs, these relatively small power consumers are a higher percentage of the total power consumption, so have more effect on efficiency.
The graph cuts off at the low end. This is because for safety reasons, we can't run our electrolyser below a certain threshold (usually 10-20%). This is related to hydrogen crossover in the cells .
So if you are looking to model or purchase an electrolyser, ensure that the manufacturer gives you the equivalent of the graph above, so you can see how the efficiency changes with output.
Why do most electrolysers have a minimum operating point (usually about 15-20% of max output)?
Safety is the main driver. Most pressurised electrolysers operate at ~15-30bar H2 pressure with only a thin membrane / diaphragm separating H2 from O2, and as most people will be aware, hydrogen diffuses very easily through materials. As a consequence, when the hydrogen system is pressurised, there is a slow, but steady flow of hydrogen to the oxygen (anode) side. This is a significant problem, as 4% H2 in O2 is explosive, and as such, we aim to keep it below 2%.
Fortunately, when the electrolyser is running hard, it generates a significant amount of oxygen, which dilutes the H2 on the anode below dangerous levels. But, as you turn down the electrolyser, the H2 crossover remains largely unaffected, but the O2 generation is reduced, decreasing the dilution and increasing the H2 concentration.
The minimum operating point is the lowest level that the manufacturers are confident that there is sufficient O2 generation to keep H2 diluted and safe – below this level could be dangerous.
So higher pressure H2 leads to a higher minimum operating point. A higher maximum current density moves the minimum point lower, as does using thicker (less efficient) membranes. If your electrolyser is operated binary (100% or off), the minimum point doesn’t matter, so you can have thinner membranes and be more efficient. However, if you take the same thin membraned electrolyser and link to PV, it would require a high minimum operating point and you would lose hours of production at dawn and dusk. Options are to go for thicker membranes, or turn down your H2 pressure, or use a recombination catalyst or have multiple stacks and turn some off to concentrate power in the remaining. All come with down-sides.
This is also why electrolysers don’t like being held for a ‘warm start’ – i.e. pressurised hydrogen but no generation. This can only happen if manufacturers are happy they have purged the anode side (usually with N2) before and after the hold.
***
More about HYDErogen
HYDErogen can offer technical due diligence services, help solve real-world problems, and is backed by an engineering sister-company to produce original equipment. Contact Kris Hyde on LinkedIn.